Chemistry
Saccharin is a cyclic sulfimide, usually used as the sodium salt.
Molecular formula:
C7H5NO3S (saccharic acid)
C7H4NNaO3S (sodium saccharin)
Molecular weight:
183.18 (saccharic acid)
205.17 (sodium saccharin)
Taste
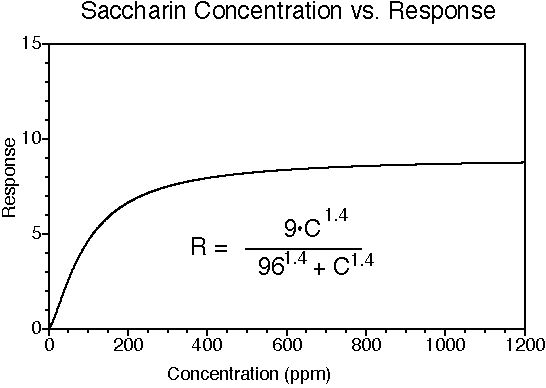
Saccharin has a sweet taste; many (but not all) people experience a bitter-metallic aftertaste. Its onset of sweetness is rapid. The sweetness potency relative to sucrose is about 300, but depends upon the concentration of sucrose which is being matched. The concentration vs. response relationship in water is shown below (results in food systems will vary). This graph is based on data from DuBois, Walters, Schiffman, Warwick, Booth, Pecore, Gibes, Carr & Brands in "Sweeteners: Discovery, Molecular Design, and Chemoreception," D.E. Walters et al., Eds., American Chemical Society, 1991. The equation allows you to calculate sweetness response (R) for any concentration (C). The units of R are percent sucrose equivalent; the units of C are parts per million (ppm). The saccharin data were fit to a Hill-type equation (exponential).
Safety
Saccharin (as the sodium or calcium salt) is quite water soluble. It is absorbed in the stomach (the acidic environment in the stomach neutralizes the salt to the free acid form). It is rapidly excreted in the kidneys. Saccharin has been shown to produce urinary bladder tumors in rats, and this led to a ban in some countries and a proposed ban in the US. Rather than banning saccharin, the US government required that products containing saccharin must bear a warning label.
The International Agency for Research on Cancer has found that a combination of sodium saccharin with high pH, high calcium phosphate concentration, and high protein concentration (which is unique to rats) causes formation of a solid material in the rat bladder[1]. This material damages the rat's bladder and provokes overactive regrowth of bladder cells. This phenomenon has not been observed in mice or in any other species, including humans. Thus, it is likely that saccharin carcinogenic only in rats, and that it is quite safe for humans. See my essay on saccharin and cancer .
Discovery
There is some controversy over the discovery of saccharin. Constantine Fahlberg was working in the laboratory of Ira Remsen at Johns Hopkins University. According to Fahlberg's account, he accidentally spilled some laboratory material on his hand, and noticed the sweet taste later in the evening when he was eating dinner. Fahlberg and Remsen published the work jointly [Fahlberg, C.; Remsen, I. Ueber die Oxydation des Orthotoluolsulfamids. Chem. Ber. 12:469-473 (1879)].
Later Fahlberg patented saccharin without including Remsen on the patent. Fahlberg went on to become wealthy. Remsen went on to become the president of Johns Hopkins University, and he commented "Fahlberg is a scoundrel. It nauseates me to hear my name mentioned in the same breath with him."