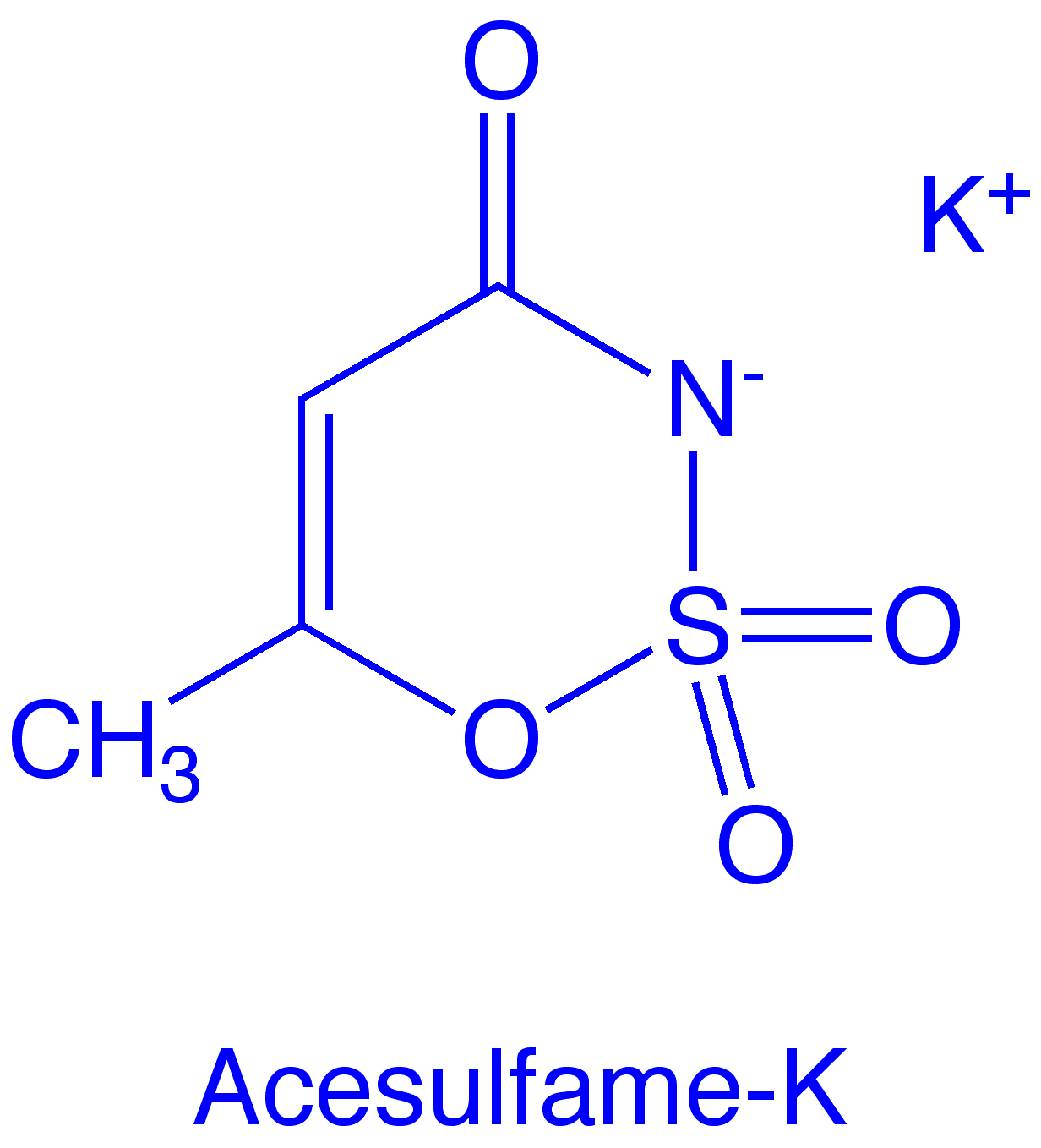
Chemistry
Molecular formula: C4H4KNO4S
Molecular weight: 201.24
Acesulfame is an oxathiazinone dioxide. Chemically, it bears some structural resemblance to saccharin. The hydrogen atom on the nitrogen is quite acidic (pKa ~2) and it readily forms salts. The sweetener is sold as the potassium salt.
Taste
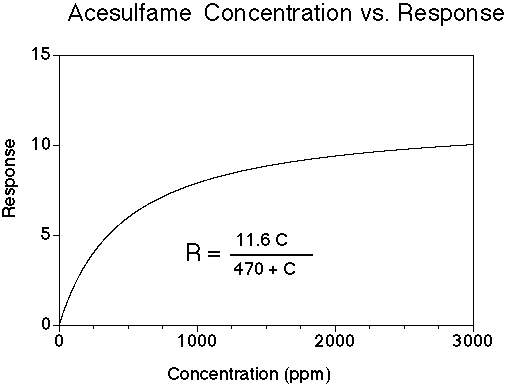
Acesulfame has a sweet taste; many people experience a bitter-metallic aftertaste (much like saccharin). Its onset of sweetness is rapid. The sweetness potency relative to sucrose is about 200, but depends upon the concentration of sucrose which is being matched. The concentration vs. response relationship in water (results in food systems will vary) is shown below. This graph is based on data from DuBois, Walters, Schiffman, Warwick, Booth, Pecore, Gibes, Carr & Brands in "Sweeteners: Discovery, Molecular Design, and Chemoreception," D.E. Walters et al., Eds., American Chemical Society, 1991. The units of R are percent sucrose equivalent; the units of C are parts per million (ppm).
Safety
FDA and JECFA have established an acceptable daily intake (ADI) of 15 mg per kg of body weight for acesulfame-K. This is approximately equivalent to 6 cans of diet soft drink. The European Food Safety Authority has set the ADI at 9 mg per kg of body weight.
Discovery
Acesulfame was discovered by a chemist, Karl Clauss, in 1967. He noticed a sweet taste when he licked his finger to pick up a piece of paper in the laboratory [Clauss, K.; Jensen, H. Oxathiazinone Dioxides--A New Group of Sweetening Agents. Angew. Chem. Internatl. Ed. Engl. 1973, 12, 869-876].